
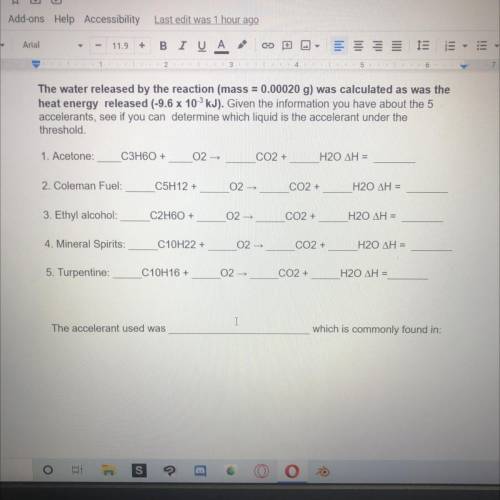
The water released by the reaction (mass = 0.00020 g) was calculated as was the
heat energy released (-9.6 x 10 kJ). Given the information you have about the 5
accelerants, see if you can determine which liquid is the accelerant under the
threshold.
1. Acetone:
C3H60+
02-
CO2 +
H20 ΔΗ =
2. Coleman Fuel:
C5H12 +
02-
CO2 +
H20 ΔΗ =
3. Ethyl alcohol
C2H60 +
02 -
CO2 +
H20 ΔΗ =
4. Mineral Spirits:
C10H22 +
02-
CO2 +
H20 AH =
5. Turpentine:
C10H16 +
02 -
CO2 +
H20 ΔΗ =
The accelerant used was
I
which is commonly found in:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3


Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3

You know the right answer?
The water released by the reaction (mass = 0.00020 g) was calculated as was the
heat energy releas...
Questions

Mathematics, 29.04.2021 07:30

Chemistry, 29.04.2021 07:30

Physics, 29.04.2021 07:30

Mathematics, 29.04.2021 07:30


History, 29.04.2021 07:30

Mathematics, 29.04.2021 07:30




Mathematics, 29.04.2021 07:30


Chemistry, 29.04.2021 07:30

English, 29.04.2021 07:30

History, 29.04.2021 07:30

Physics, 29.04.2021 07:30



Mathematics, 29.04.2021 07:30



