
Chemistry, 28.04.2021 21:30 bevanscory123
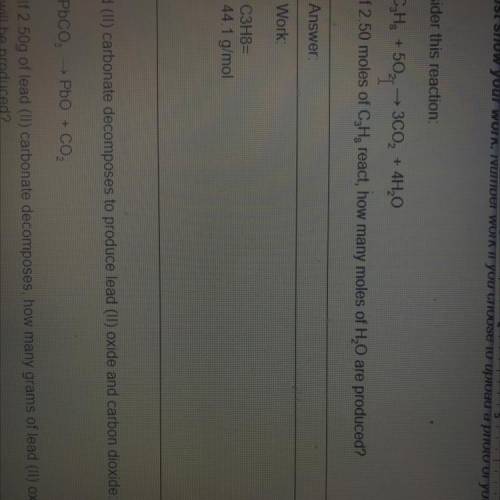
C3H8, + 5O2
+ 5O2,3CO2 + 4H20
If 2.50 moles of C3H8react, how many moles of H20 are produced?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
C3H8, + 5O2
+ 5O2,3CO2 + 4H20
If 2.50 moles of C3H8react, how many moles of H20 are produced...
If 2.50 moles of C3H8react, how many moles of H20 are produced...
Questions



Mathematics, 03.06.2020 14:58

History, 03.06.2020 14:58

English, 03.06.2020 14:58


Arts, 03.06.2020 14:58



Computers and Technology, 03.06.2020 14:58

Mathematics, 03.06.2020 14:58

Mathematics, 03.06.2020 14:58



Mathematics, 03.06.2020 14:58

Mathematics, 03.06.2020 14:58

Social Studies, 03.06.2020 14:58

English, 03.06.2020 14:58






