
Chemistry, 28.04.2021 20:40 gigimasters71p7tc6l
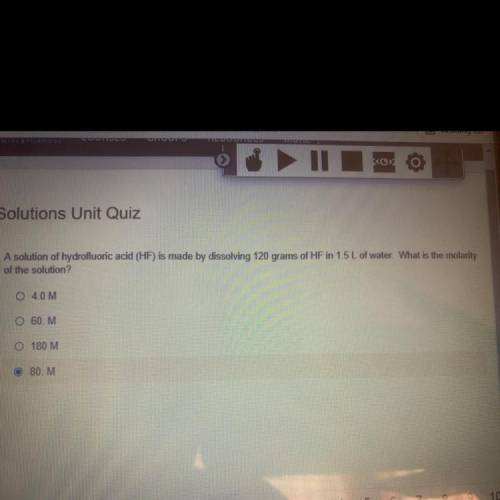
A solution of hydrofluoric acid (HF) is made by dissolving 120 grams of HF in 1.5 L of water. What is the molarity
of the solution?
HELP ASAP!!!

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
A solution of hydrofluoric acid (HF) is made by dissolving 120 grams of HF in 1.5 L of water. What i...
Questions



Biology, 04.02.2020 12:51


Chemistry, 04.02.2020 12:51

Mathematics, 04.02.2020 12:51

Mathematics, 04.02.2020 12:51

Social Studies, 04.02.2020 12:51

Chemistry, 04.02.2020 12:51




Mathematics, 04.02.2020 12:51

History, 04.02.2020 12:51


Mathematics, 04.02.2020 12:51




Mathematics, 04.02.2020 12:51



