Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
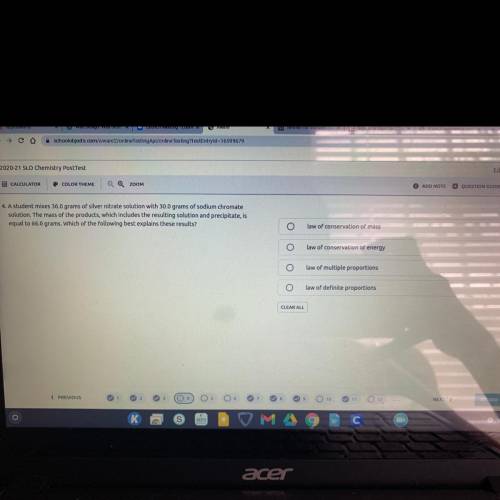
A student mixes 36.0 grams of silver nitrate solution with 30.0 grams of sodium chromate solution. T...
Questions

Mathematics, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01

Arts, 06.05.2020 15:01


Mathematics, 06.05.2020 15:01

Chemistry, 06.05.2020 15:01


Social Studies, 06.05.2020 15:01


Mathematics, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01


Mathematics, 06.05.2020 15:01

English, 06.05.2020 15:01



Biology, 06.05.2020 15:01

Mathematics, 06.05.2020 15:01