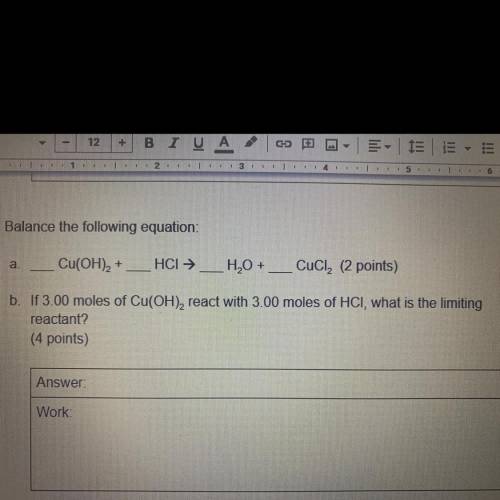
A.
Cu(OH)2 +
HCI →
H2O + __ CuCl2 (2 points)
-
b. If 3.00 moles of Cu(OH),...

Chemistry, 28.04.2021 20:30 amanuelwold
A.
Cu(OH)2 +
HCI →
H2O + __ CuCl2 (2 points)
-
b. If 3.00 moles of Cu(OH), react with 3.00 moles of HCI, what is the limiting
reactant?
(4 points)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Questions

SAT, 05.05.2021 19:10


History, 05.05.2021 19:10

History, 05.05.2021 19:10



Chemistry, 05.05.2021 19:10

Mathematics, 05.05.2021 19:10

Mathematics, 05.05.2021 19:10


Mathematics, 05.05.2021 19:10

Mathematics, 05.05.2021 19:10

Mathematics, 05.05.2021 19:10


English, 05.05.2021 19:10

Mathematics, 05.05.2021 19:10



Mathematics, 05.05.2021 19:10

Mathematics, 05.05.2021 19:10



