
Chemistry, 28.04.2021 19:50 loganharper992
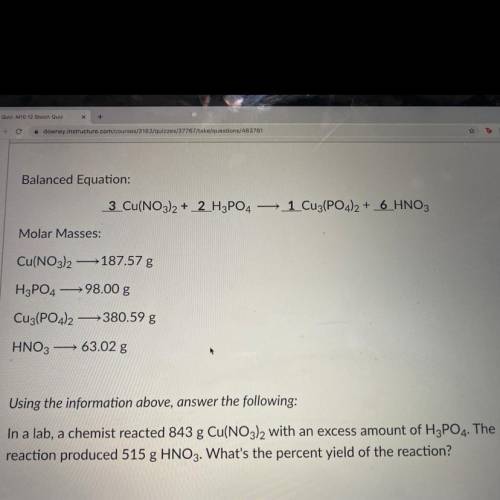
In a lab, a chemist reacted 843 g Cu(NO3)2 with an excess amount of H3PO4. The reaction produced 515 g HNO3. What's the percent yield of the reaction?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
In a lab, a chemist reacted 843 g Cu(NO3)2 with an excess amount of H3PO4. The
reaction produced 5...
Questions


Health, 05.12.2019 18:31



Chemistry, 05.12.2019 18:31


Mathematics, 05.12.2019 18:31




Mathematics, 05.12.2019 18:31

Mathematics, 05.12.2019 18:31

Physics, 05.12.2019 18:31

Mathematics, 05.12.2019 18:31


English, 05.12.2019 18:31

Mathematics, 05.12.2019 18:31

History, 05.12.2019 18:31

Biology, 05.12.2019 18:31

Mathematics, 05.12.2019 18:31



