
Chemistry, 28.04.2021 18:30 nickeymcorrea
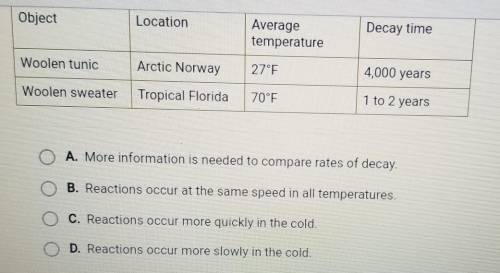
Outdoor decay of objects is a chemical reaction. Some ancient objects have been preserved extremely well in Arctic conditions. As glaciers have melted, archaeologists have discovered 4,000-year-old Viking clothing. Clarissa left her wool sweater outside at her family's summer cottage in Florida. When she returned the following summer, the sweater was in shreds, decayed by reactions that break down wool. Based on the data in the table, what can you conclude about the rate at which clothing decays in cold temperatures compared with the rate at which clothing decays in warm temperatures?
A. More information is needed to compare rates of decay
B. Reactions occur at the same speed in all temperatures
C. Reactions occur more quickly in the cold
D. Reactions occur more slowly in the cold.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1
You know the right answer?
Outdoor decay of objects is a chemical reaction. Some ancient objects have been preserved extremely...
Questions

Physics, 15.01.2021 16:10




Biology, 15.01.2021 16:10


Mathematics, 15.01.2021 16:10

Mathematics, 15.01.2021 16:10




Mathematics, 15.01.2021 16:10

Chemistry, 15.01.2021 16:10


Computers and Technology, 15.01.2021 16:10

Mathematics, 15.01.2021 16:10



Mathematics, 15.01.2021 16:10

English, 15.01.2021 16:20



