Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3


Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
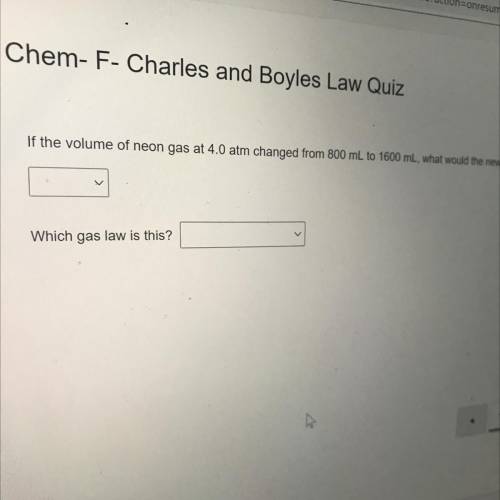
If the volume of neon gas at 4.0 atm changed from 800 mL to 1600 mL, what would the new pressure be?...
Questions




Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

Chemistry, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00


Mathematics, 18.12.2020 01:00


Biology, 18.12.2020 01:00


Biology, 18.12.2020 01:00

History, 18.12.2020 01:00

Mathematics, 18.12.2020 01:00


Computers and Technology, 18.12.2020 01:00

English, 18.12.2020 01:00

World Languages, 18.12.2020 01:00