
Chemistry, 28.04.2021 03:00 donaji1024perez
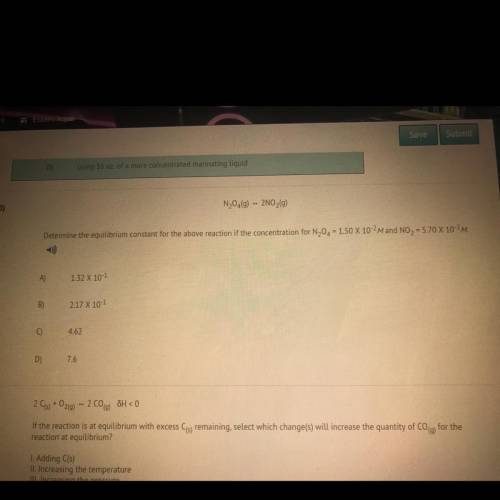
Determine the equilibrium constant for the above reaction if the concentration for N20 = 150 X 10-2M and NO2 = 5.70 X 10-2M.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1
You know the right answer?
Determine the equilibrium constant for the above reaction if the concentration for N20 = 150 X 10-2M...
Questions

Mathematics, 16.04.2020 13:20

Mathematics, 16.04.2020 13:20


Law, 16.04.2020 13:20



World Languages, 16.04.2020 13:20


Chemistry, 16.04.2020 13:20

History, 16.04.2020 13:21

Chemistry, 16.04.2020 13:21



Mathematics, 16.04.2020 13:21


Chemistry, 16.04.2020 13:21



Business, 16.04.2020 13:21

Biology, 16.04.2020 13:21



