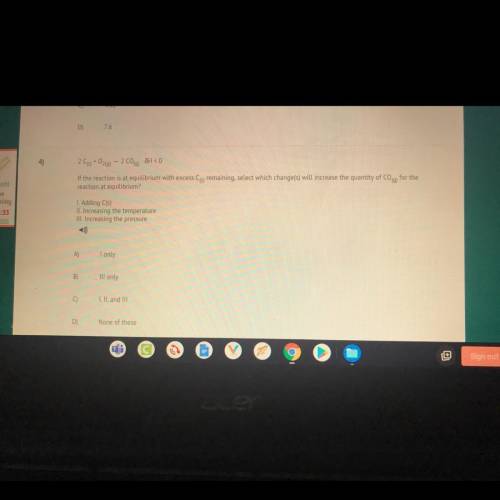
4)
209+0260) - 2 C00) JH < 0
If the reaction is at equilibrium with excess Cs remaining,...

Chemistry, 28.04.2021 02:50 mahagonylabeyta
4)
209+0260) - 2 C00) JH < 0
If the reaction is at equilibrium with excess Cs remaining, select which change(s) will increase the quantity of Coq) for the
reaction at equilibrium?
I. Adding C(s)
II. Increasing the temperature
III. Increasing the pressure

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3


Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
Questions

Mathematics, 04.06.2020 18:59


Mathematics, 04.06.2020 18:59

Mathematics, 04.06.2020 18:59

Mathematics, 04.06.2020 18:59


History, 04.06.2020 18:59

Mathematics, 04.06.2020 18:59








Social Studies, 04.06.2020 18:59

Chemistry, 04.06.2020 18:59





