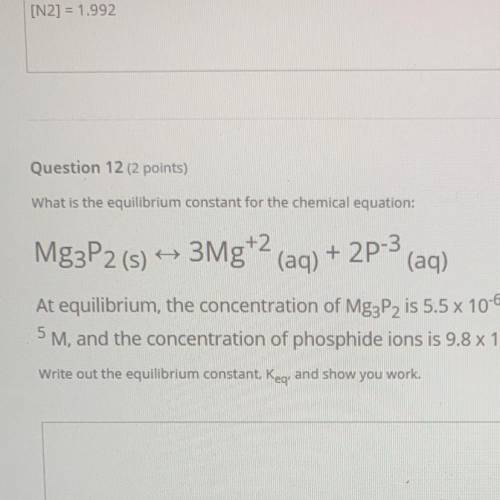
What is the equilibrium constant for the chemical equation:
Mg3P2 (s) ++ 3Mg+2 (aq) + 2P-3
(...

What is the equilibrium constant for the chemical equation:
Mg3P2 (s) ++ 3Mg+2 (aq) + 2P-3
(aq)
At equilibrium, the concentration of Mg3P2 is 5.5 x 10-6 M, the concentration of magnesium ions is 7.2 x 10
5 M, and the concentration of phosphide ions is 9.8 x 10-8 M.
Write out the equilibrium constant, Keg. and show you work.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

You know the right answer?
Questions


Mathematics, 10.03.2022 04:20

Chemistry, 10.03.2022 04:20


Geography, 10.03.2022 04:30

Mathematics, 10.03.2022 04:30

Mathematics, 10.03.2022 04:30

Mathematics, 10.03.2022 04:30

Mathematics, 10.03.2022 04:30

English, 10.03.2022 04:30

History, 10.03.2022 04:30

Spanish, 10.03.2022 04:30

History, 10.03.2022 04:30



Social Studies, 10.03.2022 04:30



Social Studies, 10.03.2022 04:30



