
Chemistry, 27.04.2021 20:50 freshysans4
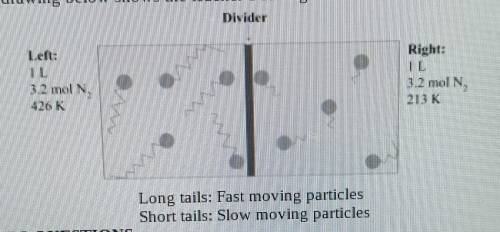
A teacher sets up a box containing 2 identical halves by inserting a divider in the middle of the box. Both the box and the divider are made of an insulating material that does not absorb or give 3.2 moles of N² gas. The temperature of the gas in the left compartment is 426 K, and in the right one 213 K. The drawing below shows the teacher's setting.
Answer The 3 Questions:
1.What is the gas pressure in the left chamber
2.The pressure in the right chamber is half the pressure of the left chamber. Explain why this is so using the Kinetic Theory of Glases.
3.What do you estimate will be the final gas temperature after removing the divider? Explain your results using the Kinetic Theory of Gases.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

You know the right answer?
A teacher sets up a box containing 2 identical halves by inserting a divider in the middle of the bo...
Questions

Mathematics, 13.07.2021 01:00

Mathematics, 13.07.2021 01:00



History, 13.07.2021 01:00


Mathematics, 13.07.2021 01:00

Spanish, 13.07.2021 01:00



Mathematics, 13.07.2021 01:10


English, 13.07.2021 01:10



Mathematics, 13.07.2021 01:10


Chemistry, 13.07.2021 01:10

Mathematics, 13.07.2021 01:10

Biology, 13.07.2021 01:10



