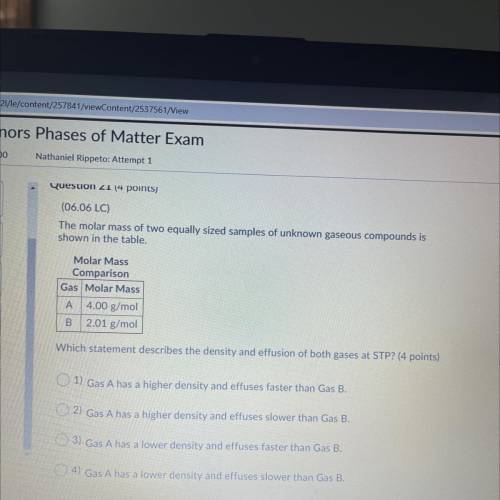
(06.06 LC)
The molar mass of two equally sized samples of unknown gaseous compounds is
shown...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
Questions


Chemistry, 18.08.2021 20:20


Mathematics, 18.08.2021 20:20


Health, 18.08.2021 20:20

Mathematics, 18.08.2021 20:20


Mathematics, 18.08.2021 20:20


Mathematics, 18.08.2021 20:20

Mathematics, 18.08.2021 20:20






Mathematics, 18.08.2021 20:20

Biology, 18.08.2021 20:20

Mathematics, 18.08.2021 20:20