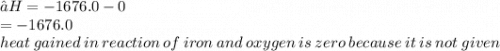2Al(S)+1.5O2(S)→Al2O3(s) H= -1676.0 KJ
2Fe + 1.5O2 (S) →Fe2O3(S)
Then the enthalpy cha...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2


Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
Questions

Mathematics, 18.03.2021 01:40


Arts, 18.03.2021 01:40



Mathematics, 18.03.2021 01:40









English, 18.03.2021 01:40





Mathematics, 18.03.2021 01:40