
Chemistry, 27.04.2021 04:10 janeou17xn
need some help with just one question please help. What is the pH of a 9.7 x 10-5 M NaOH solution? (2 formulas)
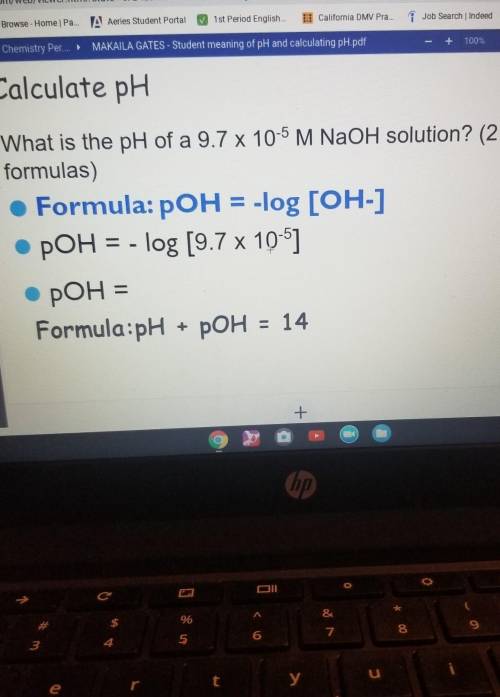

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
need some help with just one question please help. What is the pH of a 9.7 x 10-5 M NaOH solution? (...
Questions



Social Studies, 10.03.2021 08:00


Physics, 10.03.2021 08:00

Mathematics, 10.03.2021 08:00

Mathematics, 10.03.2021 08:00

Mathematics, 10.03.2021 08:00

History, 10.03.2021 08:00

English, 10.03.2021 08:00

Mathematics, 10.03.2021 08:00

Mathematics, 10.03.2021 08:00

Geography, 10.03.2021 08:00

Spanish, 10.03.2021 08:00



Spanish, 10.03.2021 08:00

English, 10.03.2021 08:00

Physics, 10.03.2021 08:00



