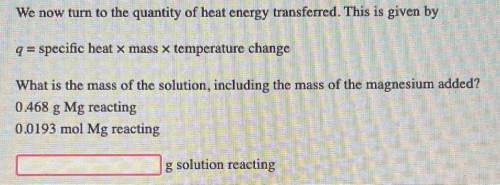Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
We now turn to the quantity of heat energy transferred. This is given by
q = specific heat x mass...
Questions


Biology, 02.08.2019 10:20

Mathematics, 02.08.2019 10:20

History, 02.08.2019 10:20


Mathematics, 02.08.2019 10:20

English, 02.08.2019 10:20


Mathematics, 02.08.2019 10:20

Biology, 02.08.2019 10:20

German, 02.08.2019 10:20


Chemistry, 02.08.2019 10:20

History, 02.08.2019 10:20

Mathematics, 02.08.2019 10:20

Mathematics, 02.08.2019 10:20

Mathematics, 02.08.2019 10:20

Biology, 02.08.2019 10:20


Mathematics, 02.08.2019 10:20