Please help
The thermochemical equation for the combustion of propane gas is:
CH4 (g)...

Chemistry, 27.04.2021 02:10 coolgirl5679
Please help
The thermochemical equation for the combustion of propane gas is:
CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (I), ΔH = -890 kJ/mol
Calculate much heat is released when 3.5 moles of propane have a combustion reaction.
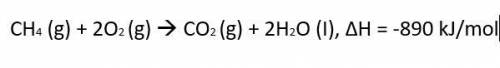

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
Questions


Mathematics, 05.03.2021 03:30


Mathematics, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30




Mathematics, 05.03.2021 03:30



Mathematics, 05.03.2021 03:30

Biology, 05.03.2021 03:30






Mathematics, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30



