
Chemistry, 27.04.2021 01:00 angellll4455
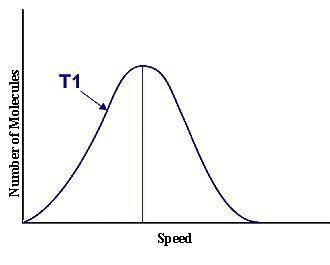
This graph represents a population of molecules in a gas versus the distribution of the average velocity(speed) of its molecules in that population. Assume all molecules to be of the same mass. In reading the graph, it is important to note three things. One, is the most probable speed is at the peak of the curve. Secondly, the most probable speed increases as the temperature increases (so shift to the right), and the distribution broadens as it increases.
On the graph, indicate the average kinetic energy of the population.
Explain your answer.
What part of the graph indicates the temperature of the sample?
Explain your answer.
Print out graph paper (click here for graph paper) and sketch a curve that represents the distribution of molecules at a temperature below the one shown. Label it as T2. Describe both T and T2 in terms of their average kinetic energy. Be specific and detailed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1


Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
This graph represents a population of molecules in a gas versus the distribution of the average velo...
Questions

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

Biology, 10.09.2020 16:01

Physics, 10.09.2020 16:01

Physics, 10.09.2020 16:01

English, 10.09.2020 16:01

French, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

History, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

English, 10.09.2020 16:01

Physics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01



