
Chemistry, 26.04.2021 23:30 itscheesycheedar
Calculate the enthalpy change for the following combustion reaction: C3H8 + 5O2 --> 3CO2 + 4H2O
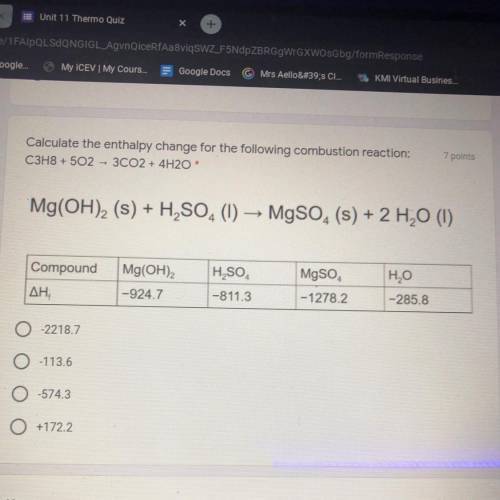

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2



Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
Calculate the enthalpy change for the following combustion reaction: C3H8 + 5O2 --> 3CO2 + 4H2O...
Questions












Computers and Technology, 16.01.2021 16:20










