
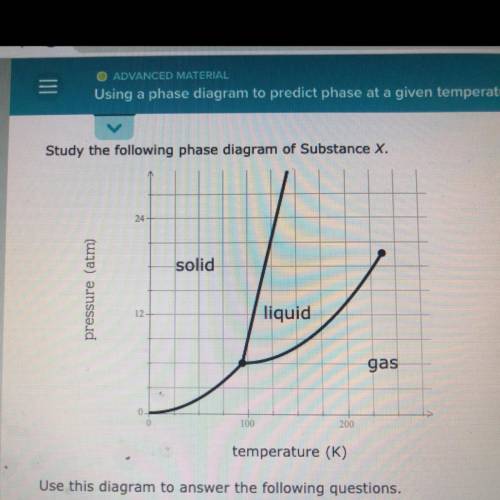
Use this diagram to answer the following questions.
Suppose a small sample of pure X is held at -48. °C and 10.7 atm.
What will be the state of the sample?
(choose one)
Suppose the temperature is held constant at -48. °C but the pressure
is increased by 3.2 atm. What will happen to the sample?
(choose one)
Suppose, on the other hand, the pressure is held constant at 10.7 atm
but the temperature is decreased by 80. °C. What will happen to the
sample?
(choose
one)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
You know the right answer?
Use this diagram to answer the following questions.
Suppose a small sample of pure X is held at -4...
Questions

Mathematics, 03.08.2021 18:40

Mathematics, 03.08.2021 18:40


Computers and Technology, 03.08.2021 18:40

Health, 03.08.2021 18:40

Mathematics, 03.08.2021 18:40

Mathematics, 03.08.2021 18:40



Computers and Technology, 03.08.2021 18:40


Biology, 03.08.2021 18:40

Mathematics, 03.08.2021 18:40


Chemistry, 03.08.2021 18:40

Mathematics, 03.08.2021 18:40

Spanish, 03.08.2021 18:40

English, 03.08.2021 18:40

Computers and Technology, 03.08.2021 18:40

Chemistry, 03.08.2021 18:40



