in terms of heat and phase

Chemistry, 26.04.2021 21:10 conyabrew82
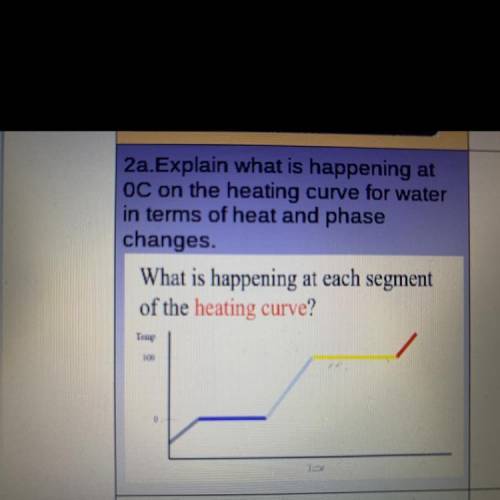
Explain what is happening at
OC on the heating curve for water
in terms of heat and phase
changes.
What is happening at each segment
of the heating curve?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What postulate of the kinetic molecular theory best explains why gases have high fluidity? because collisions between gas particles are elastic, there is no loss of energy as particles flow past each other. because gases consist of large numbers of tiny particles, they spread out and do not come in contact with each other. because the attractive forces between gas particles are negligible, gas particles can glide easily past one another. because the average kinetic energy of gas particles increases as temperature increases, gas particles behave more like a liquid. question 6 compare the compressibility of gases and liquids. support your answer by describing the arrangement of particles in gases and liquids.
Answers: 1

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
You know the right answer?
Explain what is happening at
OC on the heating curve for water
in terms of heat and phase
in terms of heat and phase
Questions

Mathematics, 18.07.2019 22:50

Physics, 18.07.2019 22:50

Mathematics, 18.07.2019 22:50

English, 18.07.2019 22:50

Chemistry, 18.07.2019 22:50

Health, 18.07.2019 22:50

Mathematics, 18.07.2019 22:50

Computers and Technology, 18.07.2019 22:50




Mathematics, 18.07.2019 22:50

Mathematics, 18.07.2019 22:50




Biology, 18.07.2019 22:50

Health, 18.07.2019 22:50

Mathematics, 18.07.2019 22:50



