
Chemistry, 26.04.2021 21:00 britotellerialuis
Laughing gas is a compound containing a ratio of
two atoms of nitrogen to one atom of oxygen.
What is the formula?
A. NO2
B. N20
c. N202
D. NO

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1


Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1
You know the right answer?
Laughing gas is a compound containing a ratio of
two atoms of nitrogen to one atom of oxygen.
Questions

Mathematics, 16.06.2021 08:50


English, 16.06.2021 08:50

Mathematics, 16.06.2021 08:50


Advanced Placement (AP), 16.06.2021 08:50

Physics, 16.06.2021 08:50


English, 16.06.2021 08:50

Mathematics, 16.06.2021 08:50



Mathematics, 16.06.2021 08:50

Mathematics, 16.06.2021 08:50

Mathematics, 16.06.2021 08:50

Mathematics, 16.06.2021 08:50

Mathematics, 16.06.2021 08:50

Mathematics, 16.06.2021 08:50


Mathematics, 16.06.2021 08:50

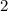 O (Nitrous oxide)
O (Nitrous oxide)

