
Chemistry, 26.04.2021 14:00 oliviaprejean18
A 9.02-g sample of liquid water at 40.3 °C is heated by the addition of 203.93 J of energy. The final temperature of the water is °C. The specific heat capacity of liquid water is 4.184 J/gK.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
A 9.02-g sample of liquid water at 40.3 °C is heated by the addition of 203.93 J of energy. The fina...
Questions


Mathematics, 20.11.2020 09:50

Mathematics, 20.11.2020 09:50


Chemistry, 20.11.2020 09:50


English, 20.11.2020 09:50


English, 20.11.2020 09:50

Mathematics, 20.11.2020 09:50

Mathematics, 20.11.2020 09:50

Mathematics, 20.11.2020 09:50


Social Studies, 20.11.2020 09:50

Mathematics, 20.11.2020 09:50


English, 20.11.2020 09:50

Mathematics, 20.11.2020 09:50


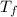 -
- 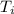
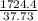 =
= 

