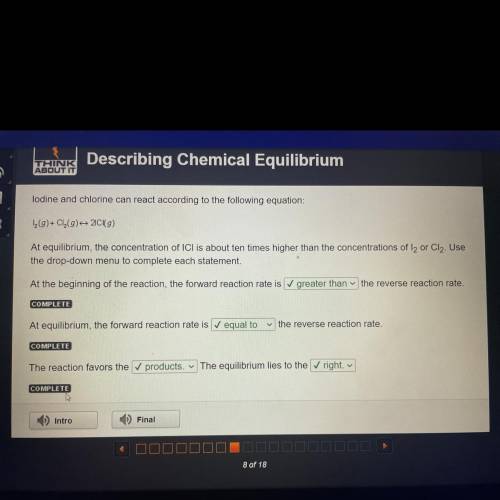
Lodine and chlorine can react according to the following equation:
1 (9)+ Cl(9) 20 (9)
At eq...

Chemistry, 26.04.2021 07:40 kinglightskin2k
Lodine and chlorine can react according to the following equation:
1 (9)+ Cl(9) 20 (9)
At equilibrium, the concentration of ICI is about ten times higher than the concentrations of l2 or Cl2. Use
the drop-down menu to complete each statement.
At the beginning of the reaction, the forward reaction rate is greater than the reverse reaction rate.
COMPLETE
At equilibrium, the forward reaction rate is equal to
the reverse reaction rate.
COMPLETE
The reaction favors the ✓ products. The equilibrium lies to the right.
COMPLETE

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Questions


Biology, 27.08.2020 17:01



History, 27.08.2020 17:01


Mathematics, 27.08.2020 17:01

Mathematics, 27.08.2020 17:01









Mathematics, 27.08.2020 17:01



History, 27.08.2020 17:01



