
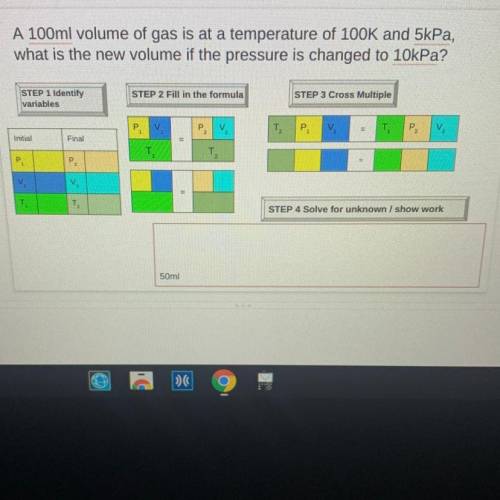
A 100ml volume of gas is at a temperature of 100K and 5kPa,
what is the new volume if the pressure is changed to 10kPa?
STEP 1 Identify
variables
STEP 2 Fill in the formula
STEP 3 Cross Multiple
P
V
T2
P
P2
Initial
Final
P.
P
=
V
V
T
TO
STEP 4 Solve for unknown / show work

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
A 100ml volume of gas is at a temperature of 100K and 5kPa,
what is the new volume if the pressure...
Questions

Health, 06.01.2021 22:20

Mathematics, 06.01.2021 22:20

Mathematics, 06.01.2021 22:20


Mathematics, 06.01.2021 22:20

Biology, 06.01.2021 22:20

Arts, 06.01.2021 22:20

Mathematics, 06.01.2021 22:20



Mathematics, 06.01.2021 22:20

History, 06.01.2021 22:20


Mathematics, 06.01.2021 22:20

Mathematics, 06.01.2021 22:20


Mathematics, 06.01.2021 22:20

Mathematics, 06.01.2021 22:20


Mathematics, 06.01.2021 22:20



