
Chemistry, 24.04.2021 14:00 joannakawata6
A gas at 1.10 atm and 30.0°C fills a flexible container with an initial volume of 2.00 L. If the temperature is raised to 80.0°C and the pressure increased to 2.40 atm, what is the new volume?
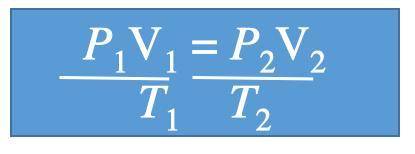

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2


Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

You know the right answer?
A gas at 1.10 atm and 30.0°C fills a flexible container with an initial volume of 2.00 L. If the tem...
Questions



Mathematics, 04.07.2019 13:00

Mathematics, 04.07.2019 13:00

History, 04.07.2019 13:00


Chemistry, 04.07.2019 13:00

History, 04.07.2019 13:00

History, 04.07.2019 13:00

Mathematics, 04.07.2019 13:00

Biology, 04.07.2019 13:00

Biology, 04.07.2019 13:00


Biology, 04.07.2019 13:00

English, 04.07.2019 13:00



English, 04.07.2019 13:00

History, 04.07.2019 13:00



