
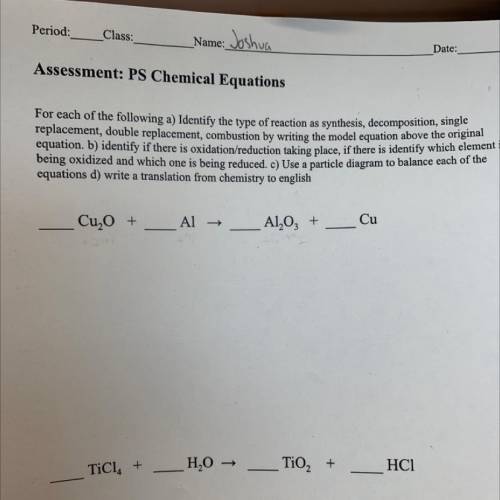
Assessment: PS Chemical Equations
For each of the following a) Identify the type of reaction as synthesis, decomposition, single
replacement, double replacement, combustion by writing the model equation above the original
equation, b) identify if there is oxidation/reduction
taking place, if there is identify which element i
being oxidized and which
one is being reduced. C) Use a particle diagram to balance each of the
equations d) write a translation from chemistry to english
Cu, O +
Al →
Al2O3 +
Cu
TiCl. +
H2O →
TiO2 +
HC1

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
How much energy moves onto the next level, in an energy pyramid
Answers: 1

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
Assessment: PS Chemical Equations
For each of the following a) Identify the type of reaction as sy...
Questions



Mathematics, 26.05.2021 18:50




Engineering, 26.05.2021 18:50

Mathematics, 26.05.2021 18:50


Chemistry, 26.05.2021 18:50





History, 26.05.2021 18:50


Computers and Technology, 26.05.2021 18:50


Mathematics, 26.05.2021 18:50



