
Chemistry, 22.04.2021 18:20 brennae8529
If the formula of ammonium nitrate is NH4NO3, determine how many moles of the solid were dissolved in the water in the experiment #2.
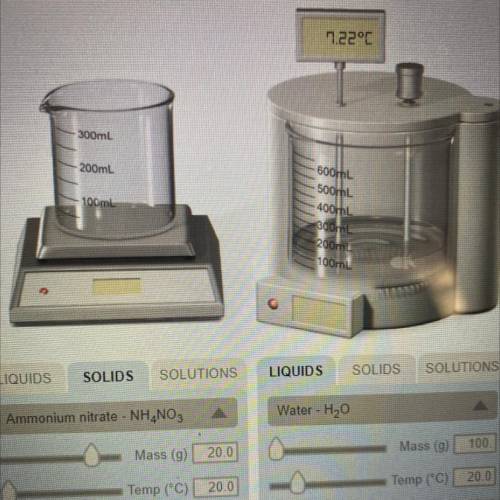

Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
If the formula of ammonium nitrate is NH4NO3, determine how many moles of the solid were dissolved i...
Questions

Chemistry, 05.05.2020 16:28





Biology, 05.05.2020 16:28

History, 05.05.2020 16:28

History, 05.05.2020 16:28


Mathematics, 05.05.2020 16:28


Physics, 05.05.2020 16:28


History, 05.05.2020 16:28

Mathematics, 05.05.2020 16:28







