2 H202 - 2 H2O + O2

Chemistry, 22.04.2021 04:40 cjasmine626
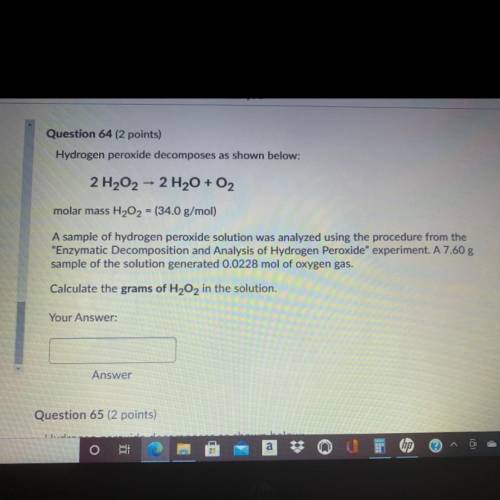
Question 64 (2 points)
Hydrogen peroxide decomposes as shown below:
2 H202 - 2 H2O + O2
molar mass H202 = (34.0 g/mol)
A sample of hydrogen peroxide solution was analyzed using the procedure from the
"Enzymatic Decomposition and Analysis of Hydrogen peroxide" experiment. A 7.60 g
sample of the solution generated 0.0228 mol of oxygen gas.
Calculate the grams of H2O2 in the solution.
Your
Answer

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 23.06.2019 08:30
Of element x has 22 protons, how many electrons does it have
Answers: 1

You know the right answer?
Question 64 (2 points)
Hydrogen peroxide decomposes as shown below:
2 H202 - 2 H2O + O2
2 H202 - 2 H2O + O2
Questions

Mathematics, 15.02.2021 20:40



Chemistry, 15.02.2021 20:40

Mathematics, 15.02.2021 20:40


Mathematics, 15.02.2021 20:40

Mathematics, 15.02.2021 20:40

Chemistry, 15.02.2021 20:40



Mathematics, 15.02.2021 20:40

Biology, 15.02.2021 20:40

Mathematics, 15.02.2021 20:40


Mathematics, 15.02.2021 20:50


Mathematics, 15.02.2021 20:50

English, 15.02.2021 20:50




