2 H202 - 2 H2O + O2

Chemistry, 22.04.2021 04:30 Alohanikolas
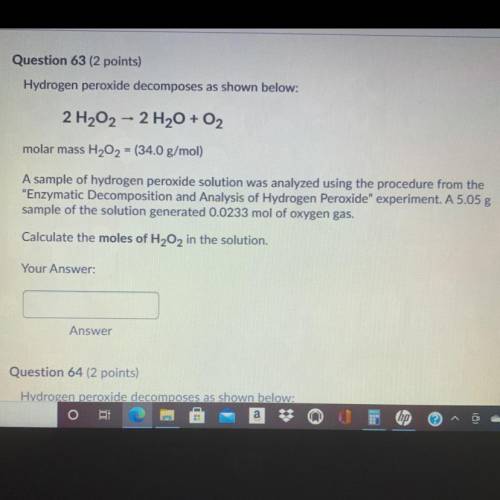
Question 63 (2 points)
Hydrogen peroxide decomposes as shown below:
2 H202 - 2 H2O + O2
molar mass H202 = (34.0 g/mol)
A sample of hydrogen peroxide solution was analyzed using the procedure from the
“Enzymatic Decomposition and Analysis of Hydrogen peroxide” experiment. A 5.05 g
sample of the solution generated 0.0233 mol of oxygen gas.
Calculate the moles of H2O2 in the solution.
Your
Answer
242 inte

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2


Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Question 63 (2 points)
Hydrogen peroxide decomposes as shown below:
2 H202 - 2 H2O + O2
2 H202 - 2 H2O + O2
Questions

Mathematics, 18.03.2021 03:20

Physics, 18.03.2021 03:20

Chemistry, 18.03.2021 03:20




Mathematics, 18.03.2021 03:20



Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20



Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

History, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20



