
Chemistry, 22.04.2021 01:20 emmanuel180
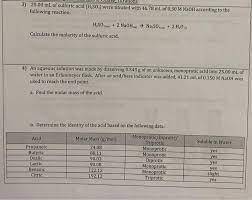
An aqueous solution was made by dissolving 0.543 g of an unknown, monoprotic acid into 25.00 mL of water in an Erlenmeyer flask. After an acid/base indicator was added, 41.21 mL of 0.150 M NaOH was used to reach the end point.
a)Find the molar mass of the acid.
b)Determine the identity of the acid based on the following data:
PLEASE HELP ME

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
An aqueous solution was made by dissolving 0.543 g of an unknown, monoprotic acid into 25.00 mL of w...
Questions

Mathematics, 04.06.2021 05:30

English, 04.06.2021 05:30

Mathematics, 04.06.2021 05:30

English, 04.06.2021 05:30


Mathematics, 04.06.2021 05:30



Advanced Placement (AP), 04.06.2021 05:30





Spanish, 04.06.2021 05:30

Mathematics, 04.06.2021 05:30


Mathematics, 04.06.2021 05:30

Mathematics, 04.06.2021 05:30


Mathematics, 04.06.2021 05:30



