
Chemistry, 22.04.2021 01:00 mivantechenko9751
A solution is prepared by dissolving 0.23 mol of chloroacetic acid and 0.27 mol of sodium chloroacetate in water sufficient to yield 1.00 L of solution. The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly. The pH does not decrease drastically because the HCl reacts with the _ present in the buffer solution. The 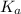 of chloroacetic acid is 1.36 x 10⁻³.
a. H₂O
of chloroacetic acid is 1.36 x 10⁻³.
a. H₂O
b. Na⁺
c. chloroacetate ion
d. chloroacetic acid
e. This is a buffer solution: the pH does not change upon addition of acid or base.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1


Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
A solution is prepared by dissolving 0.23 mol of chloroacetic acid and 0.27 mol of sodium chloroacet...
Questions

Mathematics, 21.11.2019 01:31

Mathematics, 21.11.2019 01:31

Mathematics, 21.11.2019 01:31


Mathematics, 21.11.2019 01:31



English, 21.11.2019 01:31

Mathematics, 21.11.2019 01:31


History, 21.11.2019 01:31

English, 21.11.2019 01:31

Mathematics, 21.11.2019 01:31

Social Studies, 21.11.2019 01:31

Mathematics, 21.11.2019 01:31

English, 21.11.2019 01:31

Mathematics, 21.11.2019 01:31





