
Chemistry, 21.04.2021 20:30 azibur3191
Part D
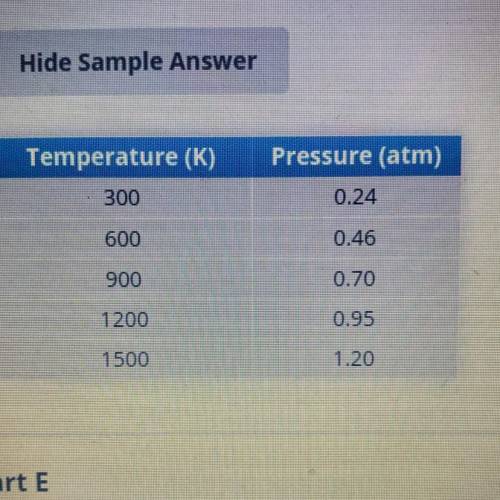
Press the yellow reset button at the bottom of the simulation screen. Under Constant Parameter, select Volume. Again,
pump the pump handle once to introduce 40 to 50 gas molecules. Record the pressure in the data table.
Use the heat control to heat the gas to each of the other temperatures in the data table, and record the new pressure.
Answer from Edmentum :)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Which of these reactions are redox reactions? check all that apply.cd + hcl → cdcl2 + h2cucl2 + na2s → 2nacl + cuscaco3 → cao + co2 2zns + 3o2 → 2zno + 2so2 ch4 + 2o2 → co2 + 2h2o
Answers: 3

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Part D
Press the yellow reset button at the bottom of the simulation screen. Under Constant Parame...
Questions

English, 16.11.2020 20:50

Spanish, 16.11.2020 20:50

Chemistry, 16.11.2020 20:50

Mathematics, 16.11.2020 20:50

Mathematics, 16.11.2020 20:50

Social Studies, 16.11.2020 20:50


Computers and Technology, 16.11.2020 20:50


Health, 16.11.2020 20:50

History, 16.11.2020 20:50

Biology, 16.11.2020 20:50

Biology, 16.11.2020 20:50





Mathematics, 16.11.2020 20:50


Mathematics, 16.11.2020 20:50



