
Chemistry, 21.04.2021 16:30 okokalyssa
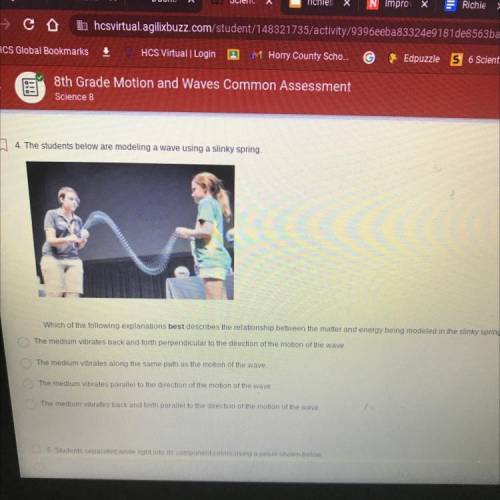
4. The students below are modeling a wave using a slinky spring
Which of the following explanations best describes the relationship between the matter and energy being modeled in the slinky spring?
The medium vibrates back and forth perpendicular to the direction of the motion of the wave.
The medium vibrates along the same path as the motion of the wave.
The medium vibrates parallel to the direction of the motion of the wave
The medium vibrates back and forth parallel to the direction of the motion of the wave

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:10
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes.which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature.sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 23.06.2019 11:40
Which of the following is true for a reliable scientific source? it cites logic. it cites opinions. it cites valid data. it cites common sense.
Answers: 2
You know the right answer?
4. The students below are modeling a wave using a slinky spring
Which of the following explanation...
Questions


Mathematics, 24.05.2021 18:30

Mathematics, 24.05.2021 18:30

History, 24.05.2021 18:30

Health, 24.05.2021 18:30


Mathematics, 24.05.2021 18:30

Mathematics, 24.05.2021 18:30



Chemistry, 24.05.2021 18:30


Mathematics, 24.05.2021 18:30

Physics, 24.05.2021 18:30

Mathematics, 24.05.2021 18:30

Mathematics, 24.05.2021 18:30

Health, 24.05.2021 18:30

Chemistry, 24.05.2021 18:30

Spanish, 24.05.2021 18:30



