Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3


Chemistry, 23.06.2019 09:00
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2
You know the right answer?
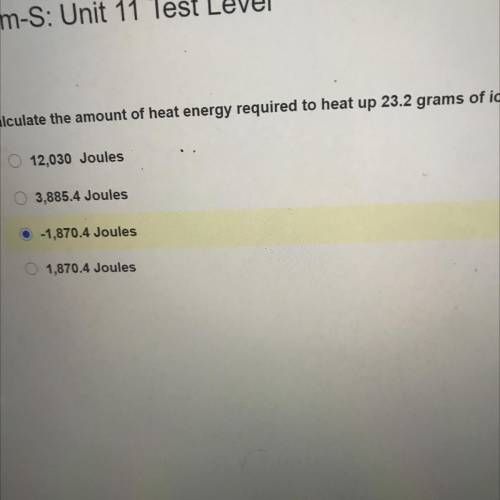
Chem-S: Unit 11 Test Level
Calculate the amount of heat energy required to heat up 23.2 grams of i...
Questions




History, 02.12.2020 17:10




Mathematics, 02.12.2020 17:10

Mathematics, 02.12.2020 17:10


English, 02.12.2020 17:10






Mathematics, 02.12.2020 17:10