Ok, i need a help.
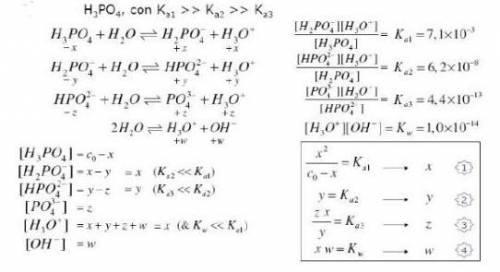
Knowing that the initial concentration of phosphoric acid
within a pool i...

Chemistry, 21.04.2021 06:50 tabisulaprinces2084
Ok, i need a help.
Knowing that the initial concentration of phosphoric acid
within a pool is 0.3mg. L-¹, and considering the equilibrium constants and the equilibrium expressions placed in the figure, determine the concentration of all specific species in the solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

You know the right answer?
Questions

Business, 01.02.2021 01:00



Social Studies, 01.02.2021 01:00



History, 01.02.2021 01:00


Biology, 01.02.2021 01:00

Mathematics, 01.02.2021 01:00


Computers and Technology, 01.02.2021 01:00

Mathematics, 01.02.2021 01:00

Mathematics, 01.02.2021 01:00

Business, 01.02.2021 01:00

Mathematics, 01.02.2021 01:00

Business, 01.02.2021 01:00

Mathematics, 01.02.2021 01:00

Mathematics, 01.02.2021 01:00

Mathematics, 01.02.2021 01:00



