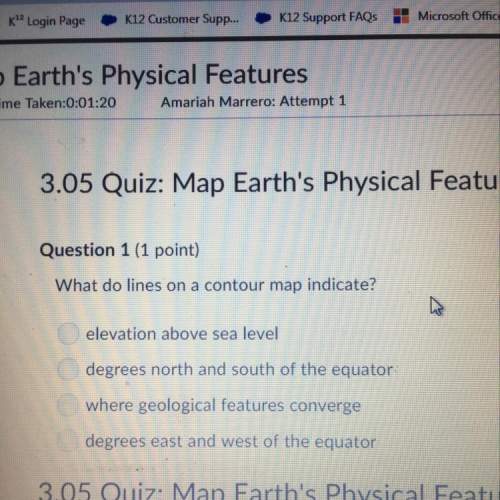
Chemistry, 21.04.2021 01:40 gensevilla54
Electrochemical cells can be used to measure ionic concentrations. A cell is set up with a pair of Zn electrodes, each immersed in ZnSO4 solution. One solution is created to be exactly 0.300 M, and the potential on its electrode is measured to be 0.045 V higher than the other electrode. What is the concentration of the other solution

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Electrochemical cells can be used to measure ionic concentrations. A cell is set up with a pair of Z...
Questions


Computers and Technology, 29.07.2019 18:10






History, 29.07.2019 18:10



Mathematics, 29.07.2019 18:10


History, 29.07.2019 18:10



History, 29.07.2019 18:10

Mathematics, 29.07.2019 18:10


English, 29.07.2019 18:10