
Chemistry, 20.04.2021 18:50 Loveekatiana
Ammonium hydrogen sulfide (NH4HS) can
decompose to ammonia and hydrogen sulfide:
NH4HS(s)<—>NH3(g)+ H2S(g)
What is the equilibrium constant expression for
this system?
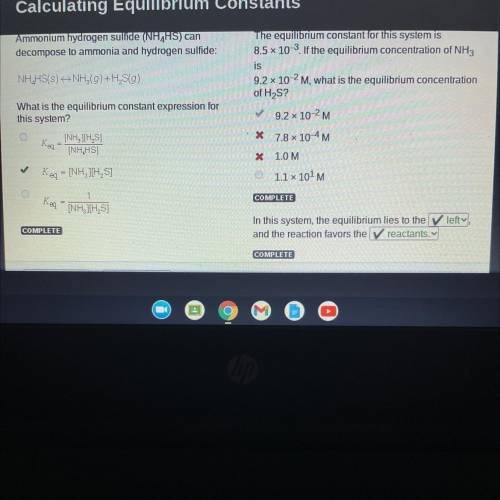

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1
You know the right answer?
Ammonium hydrogen sulfide (NH4HS) can
decompose to ammonia and hydrogen sulfide:
NH4HS(s)<...
NH4HS(s)<...
Questions

English, 16.09.2019 06:30

History, 16.09.2019 06:30

Biology, 16.09.2019 06:30

Biology, 16.09.2019 06:30

Biology, 16.09.2019 06:30

Biology, 16.09.2019 06:30

Mathematics, 16.09.2019 06:30

Chemistry, 16.09.2019 06:30


English, 16.09.2019 06:30



Mathematics, 16.09.2019 06:30

Mathematics, 16.09.2019 06:30


Chemistry, 16.09.2019 06:30

Mathematics, 16.09.2019 06:30


English, 16.09.2019 06:30

Mathematics, 16.09.2019 06:30



