
Chemistry, 20.04.2021 04:20 chutchinson256
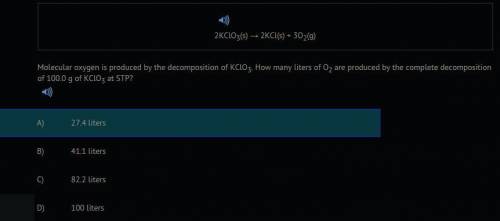
2KCLO3(s) --> 2KCL(s) + 302(g) Molecular oxygen is produced by the decomposition of KClO3. How many liters of O2 are produced by the complete decomposition of 100.0 g of KCLO3 at STP?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3
You know the right answer?
2KCLO3(s) --> 2KCL(s) + 302(g) Molecular oxygen is produced by the decomposition of KClO3. How ma...
Questions




Social Studies, 27.07.2019 02:30





History, 27.07.2019 02:30




Biology, 27.07.2019 02:30









