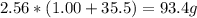2nacl + h2so4 --> 2hcl + na2so4
how many grams of hcl can be prepared from 2.00 mol h2so4...

Chemistry, 23.10.2019 03:00 julionavedo21
2nacl + h2so4 --> 2hcl + na2so4
how many grams of hcl can be prepared from 2.00 mol h2so4 and 2.56 mol nacl?
a. 7.30 g
b. 93.3 g
c. 146 g
d. 150 g
e. 196 g

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2
You know the right answer?
Questions

Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01


Biology, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

Chemistry, 18.10.2020 14:01

Biology, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01


Chemistry, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

History, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

Business, 18.10.2020 14:01

Spanish, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

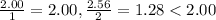 hence NaCl is the limiting reagant.
hence NaCl is the limiting reagant.