Chemistry, 29.09.2019 18:30 andrejr0330jr
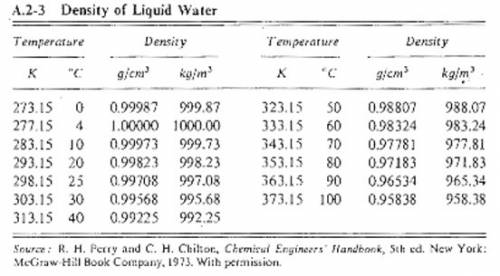
Suppose you were calibrating a 50.0 ml volumetric flask using distilled water. the flask temperature was at 20°c, and you assumed that the distilled water was as well. however, you later discover that the actual water temperature was 14°c instead. how is the mass of the 50.0 ml of distilled water you measured at 14°c different from the mass of 50.0 ml of distilled water at 20°c?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the following properties was originally used to arrange elements on the periodic table, but is no longer used to organize the modern version. which property fits this description?
Answers: 3

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2
You know the right answer?
Suppose you were calibrating a 50.0 ml volumetric flask using distilled water. the flask temperature...
Questions

English, 02.03.2021 03:40

Mathematics, 02.03.2021 03:40

Mathematics, 02.03.2021 03:40

English, 02.03.2021 03:40







Biology, 02.03.2021 03:40



Advanced Placement (AP), 02.03.2021 03:40

Mathematics, 02.03.2021 03:40

Mathematics, 02.03.2021 03:40


English, 02.03.2021 03:40