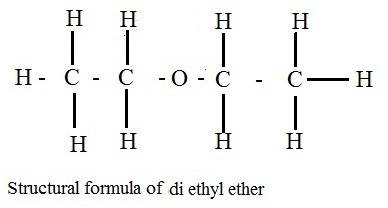Chemistry, 05.10.2019 20:00 mallardmya2006
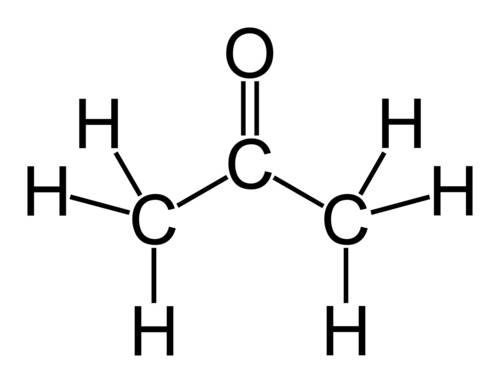
Why does ch3coch3 has stronger intermolecular forces than c2h5oc2h5? even though both have dipole-dipole as their imf, but c2h5oc2h5 has a larger molecular weight and as the molecular weight increases, the imf get stronger. so why it is the opposite here?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3

Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1
You know the right answer?
Why does ch3coch3 has stronger intermolecular forces than c2h5oc2h5? even though both have dipole-d...
Questions

SAT, 20.10.2019 10:50

Mathematics, 20.10.2019 10:50

Social Studies, 20.10.2019 10:50



English, 20.10.2019 10:50

Biology, 20.10.2019 10:50

Biology, 20.10.2019 10:50

Business, 20.10.2019 10:50


Health, 20.10.2019 10:50

Mathematics, 20.10.2019 10:50

History, 20.10.2019 10:50

Biology, 20.10.2019 10:50



Mathematics, 20.10.2019 10:50


Social Studies, 20.10.2019 10:50