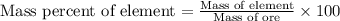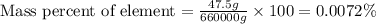Chemistry, 01.09.2019 18:30 keishah577
In 1928, 47.5 g of a new element was isolated from 660 kg of the ore molybdenite. the percent by mass of this element in the ore was:

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
In 1928, 47.5 g of a new element was isolated from 660 kg of the ore molybdenite. the percent by mas...
Questions

Mathematics, 08.12.2020 01:10

Physics, 08.12.2020 01:10



English, 08.12.2020 01:10


Chemistry, 08.12.2020 01:10


Chemistry, 08.12.2020 01:10


Biology, 08.12.2020 01:10



Mathematics, 08.12.2020 01:10





Mathematics, 08.12.2020 01:10

Mathematics, 08.12.2020 01:10