

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Given the redox reaction:
cr3+ + al--> cr + al3+
as the reaction takes place, there...
cr3+ + al--> cr + al3+
as the reaction takes place, there...
Questions


History, 24.08.2020 02:01

History, 24.08.2020 03:01


Mathematics, 24.08.2020 03:01

Mathematics, 24.08.2020 03:01

Arts, 24.08.2020 03:01



Mathematics, 24.08.2020 03:01


Computers and Technology, 24.08.2020 03:01

English, 24.08.2020 03:01

World Languages, 24.08.2020 03:01

Chemistry, 24.08.2020 03:01


Physics, 24.08.2020 03:01

History, 24.08.2020 03:01

English, 24.08.2020 03:01

Mathematics, 24.08.2020 03:01

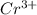
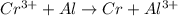
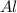 to
to 


