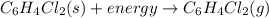Given the balanced equation representing a phase change:
c6h4cl2(s) + energy==> c6h4cl2(g)...

Given the balanced equation representing a phase change:
c6h4cl2(s) + energy==> c6h4cl2(g)
which statement describes this change?
(1) it is endothermic, and entropy decreases.
(2) it is endothermic, and entropy increases.
(3) it is exothermic, and entropy decreases.
(4) it is exothermic, and entropy increases.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3
You know the right answer?
Questions





Mathematics, 07.09.2021 16:40


Computers and Technology, 07.09.2021 16:40

Mathematics, 07.09.2021 16:40


Mathematics, 07.09.2021 16:40

English, 07.09.2021 16:40








Social Studies, 07.09.2021 16:40

Biology, 07.09.2021 16:40