Given the balanced equation:
2c + 3h2==> c2h6
what is the total number of moles of...

Chemistry, 02.09.2019 08:30 savannahvargas512
Given the balanced equation:
2c + 3h2==> c2h6
what is the total number of moles of c that must completely react to produce 2.0 moles of c2h6?
(1) 1.0 mol (3) 3.0 mol
(2) 2.0 mol (4) 4.0 mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
Questions


Mathematics, 11.11.2019 23:31

Mathematics, 11.11.2019 23:31

History, 11.11.2019 23:31

Mathematics, 11.11.2019 23:31




History, 11.11.2019 23:31



Mathematics, 11.11.2019 23:31






English, 11.11.2019 23:31

Mathematics, 11.11.2019 23:31

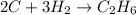
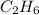 is obtained from 2 moles of C gives
is obtained from 2 moles of C gives moles of C =4 moles
moles of C =4 moles

