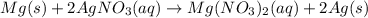Given the reaction:
mg(s) + 2 agno3(> mg(no3)2(aq) + 2 ag(s)
which type of reaction...

Chemistry, 20.08.2019 11:30 thelordoftheknowwjo4
Given the reaction:
mg(s) + 2 agno3(> mg(no3)2(aq) + 2 ag(s)
which type of reaction is represented?
(1) single replacement (3) synthesis
(2) double replacement (4) decomposition

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
Questions

English, 14.07.2019 22:00

History, 14.07.2019 22:00



Mathematics, 14.07.2019 22:00



Mathematics, 14.07.2019 22:00



History, 14.07.2019 22:00

Mathematics, 14.07.2019 22:00

History, 14.07.2019 22:00

Mathematics, 14.07.2019 22:00


Mathematics, 14.07.2019 22:00

Mathematics, 14.07.2019 22:00

Mathematics, 14.07.2019 22:00


Advanced Placement (AP), 14.07.2019 22:00