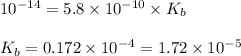Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
The acid dissociation constant for boric acid, h3bo3, is 5.8 x 10-10. calculate the dissociation con...
Questions

Biology, 14.10.2019 00:10

Health, 14.10.2019 00:10

Health, 14.10.2019 00:10

English, 14.10.2019 00:10

History, 14.10.2019 00:10

History, 14.10.2019 00:10

Mathematics, 14.10.2019 00:10

Mathematics, 14.10.2019 00:10



Mathematics, 14.10.2019 00:10






Computers and Technology, 14.10.2019 00:10

Mathematics, 14.10.2019 00:10

History, 14.10.2019 00:10

Mathematics, 14.10.2019 00:10

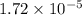
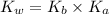
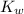 = Ionic product of water =
= Ionic product of water = 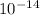
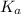 = Acid dissociation constant =
= Acid dissociation constant = 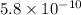
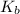 Base dissociation constant = ?
Base dissociation constant = ?