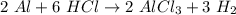Chemistry, 20.08.2019 23:50 liyahheadhigh
Which of the following products would be possible in the reaction of 2 moles of aluminum (al) with 6 moles of hydrochloric acid (hcl)? a. 2 moles of alcl2 and 1 mole of h2 b. 3 moles of alcl3 and 2 moles of h2 c. 2 moles of alcl3 and 3 moles of h2 d. 3 moles of alcl2 and 3 moles of h2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

You know the right answer?
Which of the following products would be possible in the reaction of 2 moles of aluminum (al) with 6...
Questions


Mathematics, 27.07.2020 02:01

SAT, 27.07.2020 02:01


Mathematics, 27.07.2020 02:01



History, 27.07.2020 02:01





Mathematics, 27.07.2020 02:01

Mathematics, 27.07.2020 02:01

Advanced Placement (AP), 27.07.2020 02:01

Mathematics, 27.07.2020 02:01

Mathematics, 27.07.2020 02:01