

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
One significant difference between an ionic bond, where electrons are taken from one atom and added to another atom, and a covalent or metallic bond, where electrons are shared is
Answers: 2

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
As k2o dissolves in water, the oxide ion reacts with water molecules to form hydroxide ions. write t...
Questions

Mathematics, 23.09.2021 02:00


Mathematics, 23.09.2021 02:00


Mathematics, 23.09.2021 02:00

Chemistry, 23.09.2021 02:00

Mathematics, 23.09.2021 02:00





Mathematics, 23.09.2021 02:00

Mathematics, 23.09.2021 02:00







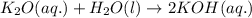
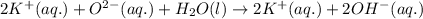
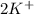 ions are present on both the sides, so the net ionic equation becomes,
ions are present on both the sides, so the net ionic equation becomes,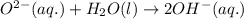
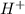 ions.
ions.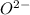 therefore it acts as an acid and oxide ion acts as a base.
therefore it acts as an acid and oxide ion acts as a base.

